How Do Animal Cells Make Energy For Cellular Processes
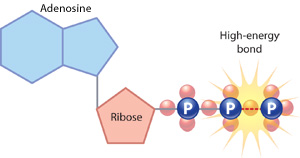
Figure 5: An ATP molecule
ATP consists of an adenosine base (blue), a ribose sugar (pink) and a phosphate chain. The loftier-energy phosphate bond in this phosphate chain is the cardinal to ATP's energy storage potential.
The detail energy pathway that a cell employs depends in large function on whether that cell is a eukaryote or a prokaryote. Eukaryotic cells use three major processes to transform the energy held in the chemic bonds of food molecules into more readily usable forms — often energy-rich carrier molecules. Adenosine 5'-triphosphate, or ATP, is the most arable free energy carrier molecule in cells. This molecule is made of a nitrogen base (adenine), a ribose sugar, and three phosphate groups. The word adenosine refers to the adenine plus the ribose sugar. The bond between the second and third phosphates is a loftier-energy bond (Figure v).
The beginning process in the eukaryotic energy pathway is glycolysis, which literally ways "sugar splitting." During glycolysis, unmarried molecules of glucose are dissever and ultimately converted into 2 molecules of a substance called pyruvate; considering each glucose contains vi carbon atoms, each resulting pyruvate contains only three carbons. Glycolysis is actually a series of x chemical reactions that requires the input of two ATP molecules. This input is used to generate four new ATP molecules, which means that glycolysis results in a net gain of ii ATPs. Ii NADH molecules are as well produced; these molecules serve as electron carriers for other biochemical reactions in the cell.
Glycolysis is an ancient, major ATP-producing pathway that occurs in almost all cells, eukaryotes and prokaryotes alike. This procedure, which is also known as fermentation, takes identify in the cytoplasm and does not require oxygen. However, the fate of the pyruvate produced during glycolysis depends upon whether oxygen is present. In the absence of oxygen, the pyruvate cannot be completely oxidized to carbon dioxide, so diverse intermediate products result. For example, when oxygen levels are depression, skeletal muscle cells rely on glycolysis to encounter their intense energy requirements. This reliance on glycolysis results in the buildup of an intermediate known as lactic acrid, which can cause a person's muscles to feel as if they are "on fire." Similarly, yeast, which is a single-celled eukaryote, produces alcohol (instead of carbon dioxide) in oxygen-scarce settings.
In contrast, when oxygen is available, the pyruvates produced past glycolysis go the input for the side by side portion of the eukaryotic energy pathway. During this stage, each pyruvate molecule in the cytoplasm enters the mitochondrion, where it is converted into acetyl CoA, a ii-carbon free energy carrier, and its tertiary carbon combines with oxygen and is released as carbon dioxide. At the same time, an NADH carrier is as well generated. Acetyl CoA then enters a pathway called the citric acid cycle, which is the 2nd major energy procedure used by cells. The 8-stride citric acid cycle generates 3 more than NADH molecules and two other carrier molecules: FADHii and GTP (Figure 6, middle).
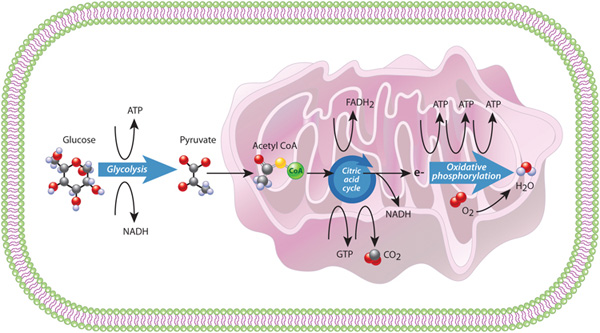
Figure 6: Metabolism in a eukaryotic cell: Glycolysis, the citric acid bicycle, and oxidative phosphorylation
Glycolysis takes place in the cytoplasm. Within the mitochondrion, the citric acrid bicycle occurs in the mitochondrial matrix, and oxidative metabolism occurs at the internal folded mitochondrial membranes (cristae).
The 3rd major procedure in the eukaryotic energy pathway involves an electron transport chain, catalyzed by several protein complexes located in the mitochondrional inner membrane. This process, called oxidative phosphorylation, transfers electrons from NADH and FADH2 through the membrane protein complexes, and ultimately to oxygen, where they combine to grade water. Equally electrons travel through the protein complexes in the chain, a slope of hydrogen ions, or protons, forms across the mitochondrial membrane. Cells harness the free energy of this proton slope to create three additional ATP molecules for every electron that travels along the concatenation. Overall, the combination of the citric acid cycle and oxidative phosphorylation yields much more free energy than fermentation - 15 times as much energy per glucose molecule! Together, these processes that occur within the mitochondion, the citric acid bike and oxidative phosphorylation, are referred to as respiration, a term used for processes that couple the uptake of oxygen and the production of carbon dioxide (Figure 6).
The electron transport chain in the mitochondrial membrane is not the only one that generates energy in living cells. In constitute and other photosynthetic cells, chloroplasts too have an electron transport chain that harvests solar energy. Even though they do not contain mithcondria or chloroplatss, prokaryotes take other kinds of energy-yielding electron send chains within their plasma membranes that also generate energy.
Source: https://www.nature.com/scitable/topicpage/cell-energy-and-cell-functions-14024533/
Posted by: thompsonmorpegir.blogspot.com

0 Response to "How Do Animal Cells Make Energy For Cellular Processes"
Post a Comment